Factors influencing rheological and textural qualities in chocolate - a review.
Cover image taken by Geoseph Domenichiello of untempered dark chocolate under electron microscope. The following is a summary of the stated research mentioned above. The content summarized here, including the figures and tables, all belong to the researchers (unless otherwise indicated). The summary attempts to stay as close to the original paper as much as possible with some adjustments in regards to jargon, length, or to focus on bean to bar aspects.
Introduction
Chocolate is a semi-solid suspension of tiny solid particles (cocoa solids and sugar) within a continuous fat phase (cocoa butter). Both the cocoa solids and cocoa butter are derived from the kernel of the seed (often referred to as a cocoa “bean”) of the Theobroma cacao fruit. Today, over 70% of the worlds production of cocoa beans is in West Africa, mostly from Ivory Coast and Ghana.
The various forms of chocolate often referred to are dark, milk, and white chocolate differ in their proportion of cacao solids, milk fat, and cocoa butter. They vary in proportion of carbohydrate, fat, and protein (Table 2).
The process of making chocolate differs according to the consumer preferences and the practices (equipment, methods) of the company making the chocolate.
Differences in the sensory properties of chocolate are due to:
different cocoa types
variations in ingredients proportions
use of milk crumb vs milk powder
blending techniques
processing methods
Fat
When it comes to the sensory character of chocolate, it’s this continuous fat phase of chocolate which influences mouthfeel and melting properties.
Because of the properties of cocoa fat (AKA cocoa butter) chocolate has that unique state of being solid at room temperature (20-25 °C) and melt at oral body temperature (37 °C). When in the mouth, the fat melts into a smooth viscous liquid with cocoa particles suspended inside (Beckett, 1999; Whitefield, 2005). The epithelia in the mouth are very sensitive to gradations of smoothness which selects for desirable lipid crystal forms.
Nutrition
Although chocolate is high in fat and often high in sugar, it may positively contribute to human nutrition due to its high levels of antioxidants. Chocolate contains polyphenols including flavonoids, primarily epicatechin, catechin, and procyanidins.
White chocolate differs from dark and milk chocolate in this regard because it lacks the cocoa solids, which is the component that contains the polyphenols. Due to lack of antioxidants, this also reduces the shelf life of white chocolate more so than dark or milk, and may go stale more quickly (Beckett, 2000; Whitefield, 2005).
Chocolate also contains minerals (potassium, magnesium, copper, iron, and more) (Holland, Welch, Unwin, Buss, & Paul, 1991).
Rheology
When chocolate melts in your mouth, the continuous fat phase of the solid chocolate changes into the continuous aqueous phase. The saliva will dissolve the sugar, while the fats and cocoa solids coat the inside of the oral epithelial surfaces (inside of the mouth). The perception of coarseness and solvation rates correspond to the particle size and work input (mastication, tongue compression, and swallowing) (Lee & Pangborn, 1986).
Particle size distribution (the various sizes of the cocoa and sugar particles) and ingredient composition influence the perception of taste (gustation) and oral volatiles (aromas) released via retronasal olfaction.
Rheological properties of chocolate are very important, and determine the chocolate’s quality in addition to flavour (Servais, Ranc, & Roberts, 2004). Chocolates with high viscosity (thick) have a pasty mouthfeel (Beckett, 2000). Viscosity is determined by composition, processing strategy, and particle size distribution. Rheological measurements give information on the sensory characteristics of chocolate.
The Initial Stages of Chocolate Manufacturing Processes
Cocoa pods reach maturity at about 4-6 months, and contain anywhere from 30-50 seeds. These seeds contain two cotyledons (oft referred to as nibs) which can be ground up to make chocolate liquor (AKA cocoa mass) or pressed to make cocoa butter and cocoa powder (Fowler, 1999; Whitefield, 2005). Complex chemical reactions during processing influence the final flavour and texture properties of chocolate (Awua, 2002; Beckett, 2000; Minifie, 1989).
Fermentation
After harvest, the fruit and seeds are fermented together for 5-7 days (Fowler, 1999). Physical and chemical changes occur which increase browning of the nibs, mainly involving reactions of polyphenols with proteins and peptides. These changes also influence future flavour of the nib.
After fermentation, the cacao beans are dried to limit mould growth during transportation and storage, and reduces bean moisture content from 60% to 8%. Sun drying is favoured for flavour development.
Roasting
Beans are roasted either before winnowing (removing the testa or husk) or after winnowing. Moisture content falls from around 8% to less than 3%. Maillard reactions occur involving amino acids (formed from fermentation protease activities which break down proteins) to yield flavour/aroma molecules with the resulting chocolate notes.
Roasting also removes much of the acids produced during fermentation, while also reducing astringency and influencing overall taste.
Grinding & Pressing
Grinding of the nib breakdown the cells and release cocoa butter which liquefies due to heat from the friction of grinding. Particle size is reduced to at least 30 microns or less. For the production of cocoa powder, fine grinding is particularly important.
When producing cocoa butter, often 78-90% of the cocoa butter can be excreted out of the nib during pressing.
Final particle size critically influences the rheological and sensory properties. A five roll refiner can be used to grind down the particles within the nib. It consists of vertical hollow cylinders which are temperature controlled. The chocolate is ground and moves between the cylinders travelling up the refiner. As the rollers shear the fragments, cocoa butter is released, and it coats new surfaces.
Conching
This determines the final texture and flavour of the chocolate, along with viscosity. A conche is a machine which agitates the chocolate at temperatures often above 50 °C for a few hours (Beckett, 2000). During conching, some remaining moisture is removed, and while it evaporates, it takes with it some unfavorable volatiles (aroma molecules). Conching times for larger scale manufacturers vary from 10-20+ hours, and range from 50-60 °C for milk chocolate and up to 70 °C for dark chocolate (Awua, 2002).
Lipid crystallization and continuous phase character during chocolate manufacture
What is “lipid crystallization”?
When liquid cocoa butter (the lipid) or liquid chocolate sets into a solid, it doesn’t just become any solid, it crystallizes. The cocoa butter solidifies based on crystal formation, similar to how a gemstone is formed.
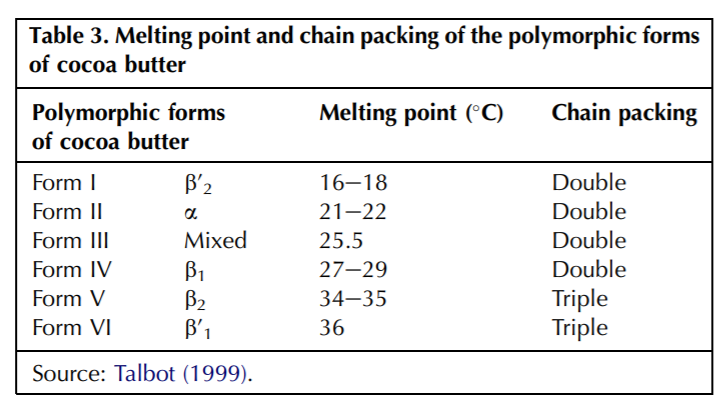
Cocoa butter can crystallize into 6 polymorphic forms, which is a function of the triglyceride composition (different combinations of various triglycerides have different properties).
Cocoa butter has 6 polymorphic forms, form V being the most desirable which results in chocolate being glossy, with a good snap when broken, contraction when solid (to help release from mould) and resistance to bloom (Beckett, 2000).
If chocolate doesn’t crystallize in form V, it likely results in form IV. In this form, reflected light is disoriented by the unstable, disorganized crystal growth which changes the colour of the chocolate and causes it to appear streaky and patchy. It is often soft and does not demould well.
Forms V and VI are the most stable, but VI is difficult to generate yourself and is only achieved through time (chocolate in form V stored for months) and eventually results in fat bloom (the whitish appearance of improperly stored chocolate). Form VI also has a much higher melting point of 36*C, and crystals are large and gritty on the tongue.
Polymorphic triglyceride forms differ in:
Distance between fatty acid chains
Angle of tilt relative to plane of chain end methyl group
Manner in which triglycerides pack in crystallization (Talbot, 1999)
How is the type of crystal formation determined?
Polymorphic form is determined by the conditions of how the liquid chocolate is cooled. Form IV (undesirable) causes the fat molecules to crystallize in a double-chain form. Form V (the ideal form) causes the fat molecules to crystallize in a triple chain form. This triple chain formation allows for closer packing of molecules and greater thermodynamic stability (doesn’t melt as easily as form IV).
Practically speaking, the formation is determined by how one tempers the liquid chocolate.
Tempering
Tempering involves pre-crystallization of a small proportion of the cocoa butter (1-3%), with crystals forming nuclei for which the remaining fat can build on to set in the correct form.
There are 4 key steps involved in tempering:
Melting to 50*C
Cooling to point of crystallization (32*C)
Crystallization (at 27*C)
Melting of any unstable crystals (at 29-31*C)
This can be achieved through various techniques. Traditionally, this was done through hand tempering by spreading melted chocolate on a cool hard surface such as granite. The seeding method is another where already tempered chocolate is added to liquid chocolate and mixed in a bowl.
Within the industry, most use various tempering machines to do the work which control for temperature and sheer (mixing). These machines consist of multistage heat exchanges where chocolate passes at different rates. Time and temperature combinations are of paramount importance.
Windhab and Mehrle found that high shear seed tempering can be beneficial as kinetics of fat crystal nucleation and polymorphic transformations are strongly accelerated by shear forces acting in high shear flow fields: overall quality of products was better, as fat bloom was reduced (Windhab et al., 2002). During tempering, temperatures were precisely controlled and agitation provided enhances nucleation rates.
Chocolate can also be tempered by the use of high pressure (Yaseda & Mochizuki, 1992) with molted chocolate compressed to 150 bar. The pressure increases the chocolate’s melting point and causes it to solidify into solid crystals of all polymorphic forms mentioned in Table 3. When the pressure is released, lower polymorphic forms melt and results in tempered chocolate.
Properties of well tempered chocolate
Good Shape
Good Colour
Gloss
Contraction From The Mould
Better Weight Control (Compact)
Stable Product
Harder
More Heat Resistant (fewer finger marks during packaging)
Long Shelf-Life
Milk Chocolate
Milk chocolate requires a slightly different regime for tempering. Due to the butter fat from the milk, the mixture of the cocoa butter fats and milk fats cause a eutectic effect. What this means is that the milk chocolate will be less susceptible to bloom, have a lower melting point (more easily melts in your hand), softening of texture, and requires a lower temperature to obtain the crystal seed (around 29.4 *C compared to 34.5 *C for dark). The two fats don’t crystallize as well as cocoa butter alone because they are different shapes and sizes, and therefore less stable. Think of trying to use Lego blocks with a generic brand of blocks, they don’t fit as nicely together.
Cocoa Butter Equivalents & Replacers
CBEs and CBRs are also used in the chocolate industry. CBEs can be used together with cocoa butter, but CBRs can only be used if all the cocoa butter is removed. CBRs also don’t require tempering, since they melt at the same temperature range as cocoa butter, but only crystallize in the V form (Talbot, 1999; Whitefield, 2005).
Shear
More recently the effect of shear on tempering has been studied such as:
Scraped surface heat exchanger with cocoa butter and chocolate (Bolliger et al., 1999)
Couette geometry with milk chocolate (Stapley, et al., 1999) and cocoa butter (Mazzanti et al., 2003)
Cone and plate system with cocoa butter (Dhonsi et al., 2006; MacMillan et al., 2002)
Parallel plate viscometer with milk chocolate (Briggs & Wang, 2004)
Helical ribbon device with cocoa butter (Toro-Vazquez et al., 2004)
Particle Size Distribution In Chocolate
Chocolate is a polydisperse suspension, meaning it contains particle sizes of various sizes. Essentially, chocolate is composed of sugar and cocoa/milk solids of various sizes suspended within cocoa butter.
Particle size distribution refers to not only the size of the particles (mainly cocoa solids and sugar) but also the variance in size and the quantity of each of the sizes. For example, what percentage of the particles are 30 microns vs 20 microns vs 10 microns. When you refine the cocoa nib, particle sizes will often get smaller with longer grinding time, but there will still be a variance of sizes nonetheless.
Beckett (2000) states that the largest particles determine mouthfeel in regards to grittiness, while the smaller particles are important for liquid chocolate flow.
Grittiness
Optimization of particle size distribution requires you to consider the sensitivity of the human palate. For example, 30 microns is usually the maximum particle size you want in your chocolate, or it will feel “gritty or coarse” in the mouth.
Generally speaking, continental Europe is described to make chocolate with a particle size of 15-22 micrometers, while in North America it tends to be 20-30 um (Jackson, 1999). However, now with globalization the traditional differences have begun to blur.
Viscosity
Particle size doesn’t just determine how “gritty” or “smooth” your chocolate is, but also has impact on the rheology of the liquid chocolate, that is, how it flows (think of water vs ketchup - different flow properties). This in turn influences the chocolate’s sensory perception (mouthfeel etc.).
A maximum particle size of only 20 microns will have a creamier taste and texture than at 30 microns. Figure 3 shows a typical particle size distribution within commercial enrobing chocolate.
As a larger particle is broken into 2 or 3 smaller particles, not only is the particle size being reduced, but the total particle surface area has now increased. The more particle surface area, the more cocoa butter is required to coat the additional surface area. Therefore, specific surface area and mean particle size influence yield stress (Beckett, 2000). The more the surface area, and the less cocoa butter to coat them, the thicker (more viscous) the chocolate becomes. This is one reason why refining your chocolate for a great deal longer may result in a slightly thicker chocolate.
So although smaller particle size is favorable to mouthfeel, it does increase the chocolate viscosity, and make it thicker, making it more difficult to mould or to send through tempering machines.
Adjusting viscosity
Aguilar and Ziegler (1995) utilized bimodal particle size distribution for a controlled reduction in viscosity. What does this mean? They used the idea of mixing chocolates together with two different particle sizes as a way to control for and reduce the viscosity.
Servais et al. (2002) blended fine (d=8.5 microns) and coarse (d=17.0 microns) particles, varied the ratio, and influenced the shear viscosity. Yield value was closely related to mean particle diameter and particle surface area. They stated a ratio of 60% coarse to 40% fine gave the lowest viscosity.
Most often, chocolate viscosity is adjusted by adding fats, usually extra cocoa butter or surfactants such as lecithin. However, this can be costly, since cocoa butter and many surfactants are quite expensive.
Thus, there are benefits to adjusting particle size distribution and playing around with the ratio of various sized particles in order to lower the viscosity. In doing so, less additional fat is needed to modify the viscosity. However, particle size distribution isn’t the only factor in determining flow.
Compositional effects on rheological and textural qualities in chocolate
The Role Of Fats
Cocoa nibs are about 55% fat (AKA cocoa butter). Cocoa butter triglycerides are made up mostly of:
Stearic Acid (34%): Saturated Fat
Palmitic Acid (27%): Saturated Fat
Oleic Acid (35%): Monounsaturated Fat
A small amount of polar lipids, sterols, and tocopherols (Talbon, 1999)
Factors such as growing conditions and origins also determines and slightly alters the fat composition of cocoa butter.
It’s the unique and simple glyceride composition that allows chocolate to melt over 23-37*C.
CBEs (cocoa butter equivalents) can be added in any proportion to chocolate with a significant impact on texture. In the EU, vegetable fats are permitted up to 5% for a product sold as chocolate (Cocoa and Chocolate Products Regulations, 2003).
CBRs (cocoa butter replacers, such as lauric fats, palm kernel oil, coconut oil) crystallize only in form, B’, in a very different way and are used to completely replace cocoa butter (Talbot, 1999). Low-caloric fats such as caprenin can also be used as CBRs. Non-lauric fats can be mixed with cocoa butter.
Most chocolates contain between 25-35% fat. Specifically designed coating chocolate such as for ice cream are much higher in fat, while some cooking chocolate or vermicelli sprinkles are lower in fat. Generally, higher quality chocolate will have higher fat content and lower particle size than something used to coat cookies.
Viscosity and Fat
The effect of adding only 1% extra cocoa butter to chocolate to alter viscosity depends on the amount of fat already present and the viscosity parameters being considered.
If the chocolate has 32% fat, adding 1% more will have very little change on viscosity. On the other hand, adding 1% more fat to chocolate with 28% fat will have a dramatic effect on the plastic viscosity, which is almost halved. The change becomes even more apparent at below 23% “chocolate” which is essentially a paste at that point (Beckett, 2000).
The Role Of Sugar
Sugar is considered an inert ingredient, really only contributing to sweetness. A change of 1-2% in sugar content has a great effect on costs, while a 5% change has an apparent impact on flavour (Beckett, 1999). It is most often used in the form of sucrose.
In milk chocolate, a small amount of lactose (a sugar) is also present. The lactose may also enhance browning when participating in the Maillard reactions (Bolenz et al., 2006; Kruger, 1999).
Monosaccharides such as glucose and fructose are rarely used due to the fact that they are difficult to dry. Sugars added to chocolate making must be dry due to fact that chocolate is based on a fat phase. The additional moisture from these sugars would increase the interactions between sugar particles (colliding instead of moving past one another), and increase viscosity.
Dextrose and lactose can successfully replace sucrose in milk chocolate (Bolenz et al., 2006; Muller, 2003).
Sugar-Free
Sugar free chocolate is becoming more popular especially among diabetics. Sugar alcohols being used include:
xylitol
sorbitol
mannitol
lactitol
However, replacing sucrose with these sugar alcohols impacts rheological properties. Sokmen and Gunes (2006) notes maltitol in chocolate has similar rheological properties to sucrose, and is recommended as a good alternative in regards to rheology. Chocolate made with isomalt resulted in higher plastic viscosity.
The EU limits consumption of sugar alcohols to 20 g per day since these sugar alcohols can have laxative effects (Kruger, 1999).
The Role Of Milk And Other Dairy Components
Since one can’t add liquid milk to chocolate, milk solids are added instead. Most often milk solids contribute between 12-25% of the chocolate. Milk contains:
5% lactose
5% milk fat
3.5% protein
0.7% minerals
Milk fat is composed of various fatty acids, but includes many of the same found in cocoa butter such as oleic, stearic, and palmitic (Haylock & Dodds, 1999). Milk fat is:
mainly liquid (15-20% solid) at ambient temperatures
softens the chocolate
slows the setting of chocolate
makes up about 30% of the fat content of milk chocolate (German & Dillard, 1998).
inhibits fat bloom
prone to oxidation and impacts shelf-life (Haylock & Dodds, 1999)
Milk proteins add to the perceived creaminess of the milk chocolate. The proteins in milk is mostly 80% caseins and 20% whey. The casein acts as a surfactant (like lecithin) and helps reduce the viscosity of chocolate. On the other hand, whey proteins increase viscosity (Haylock & Dodds, 1999).
Milk solids added as spray-dried skimmed milk powder or full cream milk powder contribute to:
flavour
texture
liquid flow properties
Milk fat freely reacts with cocoa butter when mixed with skim milk powder, but the milk fat is strongly bound in full cream milk powder. Therefore, the skim milk powder softens the cocoa butter (since the two different fats don’t stack/crystallize as well as cocoa butter alone).
In some European countries, chocolate crumb is preferred. Chocolate crumb is created when cocoa liquor (ground up nibs) is mixed with a sugar milk mass and vacuum dried, which gives it a characteristic brown colour and cooked flavour. Crumb has a longer shelf-life than milk powder since the chocolate liquor provides antioxidants (Holland et al., 1991) which protect the milk fats from going rancid (Beckett, 2000; Haylokc & Dodds, 1999).
The role of surfactants in modern chocolate confectionery
Chocolate has a continuous fat phase made up of the cocoa butter. Sugar particles are hydrophilic and lipophobic, and therefore will not dissolve in the cocoa butter. Therefore, during mixing and grinding them together, the sugar particle surfaces become coated with fat so that they can glide within the fat phase and not clump together or collide (which would increase viscosity).
Adding surface-active agents (surfactants) can be beneficial in reducing the viscosity and improving flow while allowing the fat content of the chocolate to be reduced. They attach to the sugars and allow them flow better within the cocoa butter without colliding or clumping.
The choice of natural surfactants (gums, lecithin, soluble polysaccharides) or synthetic (carboxymethyl cellulose) depends on what the chocolate will be used for (Schantz & Rohm, 2005).
Lecithin is used most often, and is a by-product of soya-oil production. It’s a mixture of natural phosphoglycerides (Minifie, 1989). In chocolate the most surface active component of crude lecithin is believed to be phosphatidylcholine (Vernier, 1998).
Lecithin greatly changes yield value and plastic viscosity when added between 0.1 and 0.3%. Not only doe sit reduce viscosity, but allows the chocolate to tolerate higher moisture levels. At more than 0.5% the yield value increases white plastic viscosity falls (Chevalley, 1999; Rector, 2000; Schantz & Rohm, 2005). An increase in the yield value causes micelle formation (basically a clump of lecithin molecules often around sugars) which hinders chocolate flow. Therefore, too much lecithin will have the reverse effect in regards to flow.
Particle size comes into play here, since chocolate with more smaller particles has more surface area to cover, and requires more lecithin to coat the sugar particles.
Lecithin can’t be added at more than 1%, but will always be present at trace levels coming from the cocoa and milk ingredients.
Polyglycerol polyricinoleate (PGPR) is obtained from polycondensation of castor oil and glycerol (Vernier, 1998). The EU allows PGPR to be used in chocolate at up to 0.5% (Rector, 2000). It doesn’t appear to impact viscosity, but can reduce yield values by up to 50% at 0.2% which turns the chocolate into a Newtonian liquid to be able to flow more easily. Chocolate with 35% cocoa butter has a similar yield value to chocolate with 32% cocoa butter and 0.1% PGPR (Rector, 2000).
Some makers combine PGPR with Lecithin in order to obtain the benefits from both (reduce yield value and viscosity).
Moisture And Chocolate Flow
Melted (AKA molten) chocolate often has a moisture content of 0.5-1.5%, mostly found in the cocoa solids. At this level, the moisture doesn’t appear to impact chocolate flow. Moisture above these levels allows the hydrophilic (water loving) sugars to aggregate and form gritty lumps. As well, the moisture on the sugar particles will increase friction as they move, and therefore impede flow and increase viscosity (thickness).
For every 0.3% extra moisture left within chocolate, the manufacturer must add an extra 1% fat (Beckett, 2000). Because cocoa butter is the most expensive ingredient, makers prefer to reduce the moisture content as much as possible.
Moisture content at 3-4% greatly increases viscosity and yield value of chocolate.
Conclusion
The physical properties of chocolate and how the chocolate behaves when melted is influenced mostly by:
processing techniques
particle size distribution
ingredient composition (how much and which type of ingredient)
To improve the quality and texture of chocolate, one can manipulate these factors mentioned above. However, we require more knowledge of particle size distribution and ingredient composition to better understand the underlying principles that affect changes in flow behaviour. Future studies require combining sensory and instrumental analysis of texture and flavour release and characterizations of effects of tempering on melting behaviour of chocolate.