Dynamics Of Volatile And Non-Volatile Compounds In Cocoa During Fermentation And Drying Processes
Introduction
Cocoa beans are classified by their overall aroma quality, which is determined according to the aromatic compounds presents. Factors which impact the overall volatile composition of the chocolate include genotype, agroclimatic conditions, fermentation, drying, and industrialization processes used.
Fermentation
During fermentation, the mucilaginous pulp (the fruit) which surrounds the seeds is removed. Heat is generated within the fermentation heap and the pH drops, both of which inhibit seed germination. The pulp is rich in sugars such as glucose, fructose, sucrose, inorganic salts, and citric acid (which gives it a pH of 3-3.5. The constituents of the pulp make it ideal for yeast and bacteria to grow.
The job of the yeast is to ferment the carbohydrates of the pulp, and produce ethanol and carbon dioxide, as well as a rise in overall temperature. Schwan and Wheals (2004) found that following yeasts:
Kloeckera apiculata
Saccharomyces cerevisiae var. chevalieri
produced large amounts of:
isopropyl acetate
ethyl acetate
1-propanol
isoamyl alcohol
2,3-butanediol
diethyl succinate
phenylethanol
These are considered desirable volatile compounds found in high quality cocoa products. At the end of alcoholic fermentation, lactic acid bacteria and acetic acid bacteria grow. Lactic acid bacteria consume glucose and citric acid from the pulp to produce lactic acid. Lagunes-Galvez et al. (2007) reported that the highest concentration of lactic acid occurred during day 5 of fermentation. Lactic acid is not considered a favorable product, and remains in the chocolate even after processing (Thompson et al., 2001). The acetic acid bacteria oxidize the ethanol and produce acetic acid and ethyl acetate. Too much acetic acid may be detrimental to cocoa quality (Brito et al., 2000). During fermentation, the acetic acid enters the bean and causes the pH to decrease from 6.5 within the bean to 4.5 (Thompson et al., 2001). If cocoa beans have a high pH of 5.5-5.8, they are considered poorly fermented. Cocoa beans with a lower pH of 4.75-5.19 are considered well fermented (Afoakwa et al., 2008). Cocoa beans with an intermediate pH of 5.20-5.49 produced chocolate with higher notes of chocolate flavour (Jinap et al., 1995).
Drying
Drying reduces the moisture of the cocoa beans to under 8%. Drying is carried out either by laying the cacao out in the sun, or artificial methods of drying. Drying reduced the acidity and astringency in the post-fermented cocoa beans. However, some authors have suggested those volatiles and non-volatiles remain after fermentation, drying, and roasting (Bailey et al., 1962; Frauendorfer et al. 2008).
This study
Principal component analysis (PCA) can be used to analyze the dynamics of volatile and non-volatile compounds during both fermentation and drying. The cocoa cultivated in Mexico has been classified as low quality at the time of this publication. There are no studies that have attempted to identify the volatile compounds producing during fermentation and drying to evaluate the quality of the cocoa beans. This research aims to identify the different volatiles (alcohols, aldehydes, ketones, esters, carboxylic acids, and pyrazines) and non-volatile (sugars and carboxylic acids) compounds during both the fermentation and drying processes using PCA.
Materials & Methods
Fermentation & Drying
Cocoa beans were obtained from Cunduacan, Tabasco, Mexico. The cocoa beans were harvested by traditional methods, and naturally fermented. They were fermented in 1000 kg masses in wooden boxes 1 meter cubed and allowed to ferment for 8 days at environmental temperature. Cocoa beans were manually turned from one box to the next once per day to ensure aeration and uniform fermentation.
Fermented beans were laid out on concrete floors in layers of 5-10 cm thick and sun dried. They were mixed manually every day for 5 days to obtain even drying.
Sample Collection
Samples of 2kg of fermented or dried cocoa beans were taken during each day of the process (8 days for fermentation, 5 days for drying). Samples for fermenting were labelled from RC0 (raw cocoa beans) and then FC1-FC8 for each fermentation day. Samples from dried cacao were labelled DC1-DC5. Sub-samples were frozen and transported to the laboratory. Half the samples were used to test for sugars and carboxylic acid compounds, and the other were analyzed for volatile compounds. The testa was manually removed to obtain the cocoa bean. The beans were then ground in a mortar and pestle until a cocoa flour was obtained and processed for freeze drying and stored at about -20°C until utilized.
Results & Discussion
Changes of pH during fermentation and drying process
The pH decreased from 6.4 at zero time (RC0) to 4.5 by day 8 during the fermentation. Similar results were found by Nazaruddin et al. (2006). Very low pH has been associated with lower notes of chocolate (Jinap et al., 1995), and Portillo et al. (2007) noted that a pH lower than 4.5 decreased the aromatic potential of cacao.
When cocoa beans are dried slowly, there is a loss of volatile acids and water along with an increase in pH (Nogales et al., 2006). In this experiment, it was found that after 2 days of drying the pH decreased significantly from 4.7 to 4.6 (P<0.05). When drying temperature is near 60°C cocoa beans can have higher concentrations of acetic, propionic, isobutyric, and isovaleric acids. During the drying time for this experiment the average temperature was 47°C.
The acidity within the cocoa beans had a significant increase from 0.0062% to 0.106% during the 8 days of fermentation. There is a high correlation between acetic and lactic acid and pH (r=0.86) titratable acidity (r=0.91), indicating that the acids could be responsible for the high values of acidity in cocoa beans (Jinap & Dimick, 1990).
Sugar and Non-Volatile Acids
Before fermentation (RC0), cocoa beans have higher concentrations of sucrose sugar and lower amounts of reducing sugars glucose and fructose (Hashim et al., 1998b). In this study, it was found that sucrose concentrations decreased significantly during fermentation, and a rise in glucose and fructose (Fig. 1a). Sucrose is converted to glucose and fructose during fermentation. It was observed in this study that glucose increased 2.7-fold and fructose increased 2.4-fold by the end of fermentation.
Organic acids were also analyzed, and at RC0 (before fermentation) citric acid concentration was higher than malic, lactic, oxalic, and succinic acids. The changes which occurred during fermentation for malic and oxalic acid were not significant. However, from beginning of fermentation until the end of drying malic acid and lactic acid concentrations did increase significantly overall. Lactic acid had the highest concentration at day 3 of fermentation (Fig. 1b). Too much lactic acid is not favorable as it produces an excessive sourness that can mask the chocolate flavour (Lagunes-Galvez et al., 2007; Thompson et al., 2001). Citric acid was depleted after fermentation and drying. Citric acid can be metabolized into acetic acid, carbon dioxide, and lactic acid (Thompson et al., 2001).
Volatile Compounds Produced During Fermentation
A total of 39 volatile compounds were identified during this study, and are shown in Table 1. Some of these compounds listed here are also responsible for producing both desirable flavours and off-flavours in cocoa beans.
Amyl alcohols are commonly found in foods, and found in the cacao fermentation process. Some of these amyl alcohols are used to evaluate cocoa flavour and fermentation degree (Oberparleiter & Ziegleder, 1997).
Six principle alcohols were identified (Fig. 2a):
· 3-methyl-1-butanol (amyl alcohol)*
· 3-methyl-2-butanol (amyl alcohol)
· 2-methyl-1-propanol
· 2, 3-butanediol*
· 1, 3-butanediol
· Phenylethyl alcohol*
These alcohols are produced during fermentation of sugars present in cocoa beans. The three alcohols with an asterisk have been reported in cacao fermentation with K. apiculate and S. cerevisiae var. chevalieri yeasts. These compounds are deemed desirable for high quality cocoa (Schwan and Wheals, 2004).
The compounds 3-methyl-1-butanol and phenylacetaldehyde have been reported as derivates from amino acids during fermentation (Afoakwa et al., 2008).
Aldehydes identified included (Fig. 2b):
· Acetoin, likely produced by alcohol fermentation from pyruvate and butanodiol (Pretorious, 2000), had the highest concentration
· Phenylacetaldehyde (Likely formed from Phenylethyl alcohol, and oxidised into the ester phenylethyl acetate [Smit et al., 2005]).
· 2, 3-butanedione
· Acetophenone
There were 7 esters found during fermentation, which ethyl acetate being the highest concentration and peaking at day 3 of fermentation (Fig. 2c). The ester 3-methyl-1-butanol acetate could be produced from the alcohol 3-methyl-1-butanol oxidation (Smit et al., 2005). Ethyl acetate is a product of esterification from acetic acid and ethanol. Acetic acid was in the highest concentrations of all the acids throughout fermentation (Fig. 2d).
Oberparleiter & Ziegleder (1997), proposed a ratio of aldehydes-amyl alcohols and acetates-amyl alcohols to evaluate fermentation degree. They suggested that cocoa beans were over-fermented if a ratio of methyl-1-butyl acetate:methyl-butanol is higher than 1.5, and tended to produce a hammy off-flavour caused by isobutyric and isovaleric acids which are formed later in the fermentation process or extended fermentation time.
In this study, the 3-methyl-1-butyl acetate:methyl-1-butanol ratio of 0.99 at 6 days of fermentation, and 1.68 at the end. According to Oberparleiter & Ziegleder, the cacao was over fermented. There was in increase of isobutyric and isovaleric acids after day four of fermentation (Fig. 2d). In retrospect, fermentation should have halted at day six when the ratio was lover than 1.5, and avoid esterification of amyl alcohols to amyl acetates. The acids propionic and butyric acid were found and would have a negative impact on cocoa quality (Serra-Bonvehi, 2005).
Volatile Compounds Produced During The Drying Process
The concentrations for 2,3-butanediol and 1,3-butanediol had the greatest concentration towards the end of drying (Fig. 3a). There was no significant change for 2-methyl-1-propanol or 3-methyl-1-butanol. Phenylethyl alcohol and benzyl alcohol showed a decrease towards the end of drying, and 3-methyl-2-butanol was not found during drying. These changes suggest that fermentation did not stop and volatile alcohol became a precursor to other compounds such as 2,3-butanediol to the aldehyde 2,3-butanedione.
Aldehyde concentrations of 2,3-butanedion, acetoin, pentanal, and phenylacetaldehyde decreased with drying (Fig. 3b). However, Tetramethylpyrazine increased during drying, and has been reported to produce aromas characteristic of cocoa and coffee and considered desirable for cocoa beans (Afoakwa et al., 2008; Serra-Bonvehi, 2005).
Drying reduces volatile acids and total polyphenols, and converts flavour precursors into pyrazines and aldehydes. Therefore, during drying flavour development continues during elimination of volatile acids and moisture (Ramli et al., 2006).
Of the Esters, 3-methyl-1-butanol acetate (likely produced from alcohol 3-methyl-1-butanol oxidation) and isobutyl acetate concentrations increased during drying (Fig. 3c). Isobutyl acetate is a precursor of isobutyric acid which can be associated with rancid, butter, cheese, and hammy aromas in cocoa beans. Ethyl acetate increased at day 2, decreased at day 3, and then increased much more by day 5. Ethyl acetate is a product of acetic acid and ethanol. This also slightly corresponded with levels of acetic acid during drying (Fig. 3d). This suggests that acid bacteria were still present after fermentation and during the drying process, producing acetic acid.
Of the acids, acetic acid concentration increased, and was much greater than all the other acids which diminished during drying (Fig. 3d). Propionic and dodecanoic acid disappeared by the end of drying. An increase in temperature and aeration favored compound volatilization of short chain volatile acids (Nogales et al., 2006).
Principal Component Analysis (PCA)
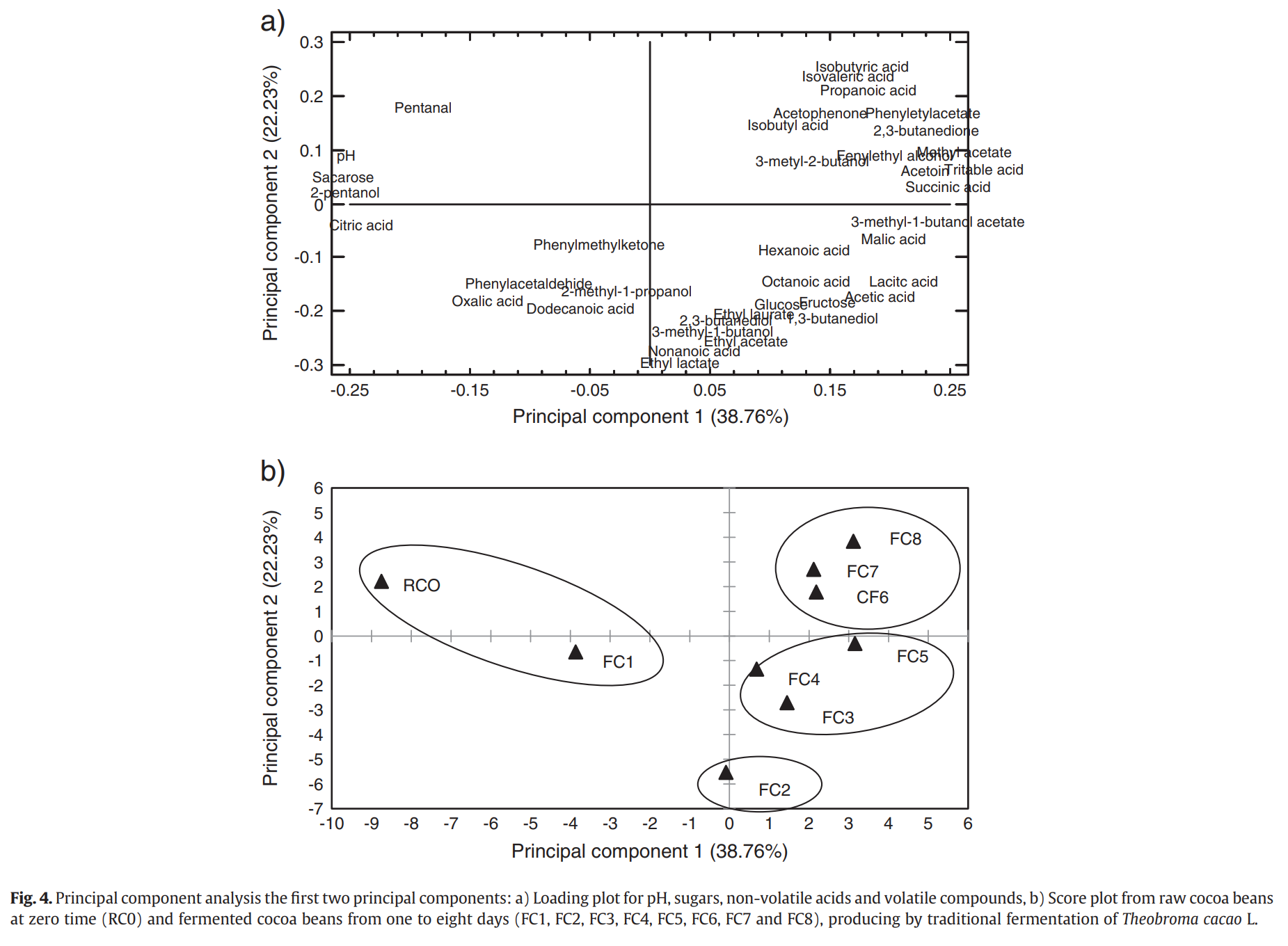
A PCA (principal component analysis) was performed to determine which the most important volatile and non-volatile compounds were found. The PCA compresses the data based on their similarities and differences. Loadings and score plots are sown in Figure 4a and 4b.
The analysis here included pH, titratable acidity, sugars, non-volatile acids (oxalic, malic, lactic, citric, and succinic) and all volatile compounds.
Fermentation
Before fermentation, the compounds with the highest concentrations were:
· Sucrose
· Citric acid
· Oxalic acid
· Dodecanoic acid
· 2-pentanol
· Phenylacetaldehyde
The concentration of these compounds decreased during fermentation. In the middle of fermentation, some compounds increased significantly such as:
· Methyl acetate
· Acetoin
· 2,3-butanedione
· Phenylethyl acetate
· 3-methyl-1-butanol acetate
· Succinic acid
· Lactic acid
· Malic acids
· Titratable acidity
Compounds with the highest concentrations overall were:
· Methyl acetate
· Acetoin
· Lactic acid
· 2,3-butanedione
At day two of fermentation, the compounds with the highest concentration were:
· Ethyl lactate
· Nonanoic acid
· Ethyl acetate
· 3-methyl-1-butanol
In the last days of fermentation, some acids increased in concentration. They included:
· Isobutyric acid
· Isovaleric acid
· Propanoic acid
· 2,3-butanediol
Drying
During the drying process, the following compounds saw a decrease in concentration:
· Phenylacetaldehyde
· Glucose
· Octanoic acid
· Phenylethyl alcohol
· Hexanoic acid
Compounds which decreased in concentration in the middle of drying were:
· Titratable acidity
· 2,3-butanedione
· Fructose
· Methyl acetate
Compounds which increased in concentration during drying were:
· 3-methyl-1-butanol acetate
· 3-methyl-2-butanol acetate
· 2-methyl-1-propanol
· Acetic acid
· Tetramethylpyrazine