Development of fat-reduced chocolate by using water-in-cocoa butter emulsions
The following is a summary of the stated research mentioned above. The content summarized here, including the figures and tables, all belong to the researchers (unless otherwise indicated). The summary attempts to stay as close to the original paper as much as possible with some adjustments in regards to jargon, length, or to focus on bean to bar aspects.
Introduction
Due to more people concerned with their weight, there is a growing market for lower calorie or lower fat foods. However, a low-fat product doesn’t have the same physical and sensorial characteristics of their full-fat counterparts. This is very true when it comes to chocolate where fat is usually one the main ingredients and is important for the characteristic snap, gloss, creamy texture, rich taste, and melt-in-mouth behaviors of the fat.
Image 1. By Bean To Bar World. You can see that the image on the right has portions of the fat (cocoa butter) replaced by water droplets encased in cocoa butter. With both chocolates being the same weight, the one on the right has some fat replaced with water, and therefore lower fat and lower in calories.
Chocolate is essentially fat (cocoa butter) with non-fat particles (cocoa powder, sugar, milk powder) suspended within it (see image 1). The fat has six polymorphic forms each with its own unique characteristics. The fifth (V) form is the most attractive to consumers.
Chocolate is also high in calorie content (about 500 kcal/100g). Some brands have introduced sugar-reduced chocolate, but the calories remain high since the fat remained unaltered or even increased (Norton et al., 2009). Developing a low-fat chocolate with a similar taste and texture to full-fat could fulfill a current need with many consumers.
Some work done on creating a low-calorie chocolate looked at substituting the cocoa butter with other ingredients (Cooper et al., 1990; Lee et al., 2009; Wu et al., 2014), reducing the sugar content (Cikrikci et al., Farzanmehr and Abbasi, 2009) or adding micronized air cells (Robert, 2006). None of these significantly altered the calorie content, or was able to have similar textural properties as regular chocolate.
Water in fat emulsion
A potential strategy to reduce calories would be to use a water-in-cocoa butter emulsion, where some of the fat is replaced by water droplets (small enough where the mouth cannot detect them).
Norton et al. (2012, 2009), Di Bari et al. (2014, 2017), and Sullo et al. (2014), have suggested the feasibility of this method producing stable emulsions with fat crystals in the polymorphic form V using the “Margarine Line” (a combination of a scraped surface heat exchanger and a pin stirrer). When a stable water-in-cocoa butter emulsion was obtained, the other ingredients were added. However, the addition of the other ingredients may break the emulsion and lead to a an undesirable texture and non-uniform product.
Norton attempted this by mixing the emulsion and other ingredients in a planetary mixer, but this method did not work. Emulsions with 20% water caused the mixture to become too viscous or crumby after the ingredients were added. Emulsions with 40% water were too soft to demould. It was thought this was caused by a non-homogeneous mixing and unable to control the temperature of the planetary mixer.
The aim of this work
To overcome the issues faced by Norton and others, and to produce a complete fat-reduced chocolate that has similar properties to a full-fat chocolate. These are the steps to be taken:
The emulsion will be optimized to identify the formulation that allows the highest fat replacement while also maintaining the highest emulsion stability.
Then part of the sugar will be added, at different concentrations, to see the effect on:
the emulsion stability and
the fat crystallization.
Finally the dry ingredients (milk powder, cocoa powder, and remaining sugar) will be added.
The emulsion and dry ingredients will be mixed in a jacketed scraped-surface vessel with temperature control in order to create a uniform product. The various samples will be analyzed in terms of:
texture
thermal behaviour
water activity.
Materials & Methods
The ingredients used were Cocoa butter (CB), polyglycerol polyricinoleate (PGPR), milk powder (MP), and cocoa powder (CP). PGPR is a surfactant, such as lecithin which is often used in the creation of commercial chocolate and more specifically milk chocolate. In this case due to the way the chocolate was made, PGPR was a more suitable than lecithin.
Pre-Emulsion Preparation
The fat was prepared by melting it in an oven at 60°C and held at this temperature overnight to remove crystal memory. PGPR was added to this fat while being agitated over a magnetic stirrer. The water was also heated to 60°C and agitated when the sugar was added.
The two phases were then mixed using an overhead stirrer (RW20 digital, IKA, Germany) for 5 minutes to make a pre-emulsion. This was then processed in the margarine line.
Emulsification Process Using The Margarine Line
Margarine, like this chocolate mixture, is also a water-in-fat emulsion. So it makes sense to use the same equipment used to make margarine to make this water-in-cocoa butter emulsion. The line consisted of two jacketed units: a scraped surface heat exchanger (A-unit) and a pin stirrer (C-unit) shown in Figure 1. Learn more about scraped surface heat exchange here. The pre-emulsion was pumped into the margarine line using a peristaltic pump at a rate of 50mL/minute. After all the mixture was processed, it was stored at 5°C.
statistical analysis
All experiments and measurements were performed in triplicate and reported as mean and standard deviation. Data were analyzed by one-way analysis of variance (ANOVA) and Turkey’s multiple comparison tests. The level of significance was defined as p ≤ 0.05.
Results & Discussion
Emulsion Optimization
The first part of the experiment was to create a stable emulsion with the highest amount of water and sugar possible. Small droplet size, no free water, and fat crystals in the V (fifth) form were also a focus.
Effect Of The Water Fraction
The first part of the experiment was to vary the percentage of water within the emulsion from 20-50% (runs 1-4, Table 1). As more water was added, droplet size also increased. This happened because the surfactant concentration remained the same (1% PGPR in each run), but the interfacial area the surfactant was to stabilize increased, which lead to larger droplets.
Cryo-SEM images show the presence of crystalline shells of fat encasing water droplets (Figure 2). Conductivity tests suggested an absence of free water, an so this was a fully-emulsified water-in-fat emulsion.
DSC analysis were performed to determine the melting properties of the prepared emulsions. When water was increased to 40%, the melting point was in the range of 30-34°C, typical of polymorph V. When water was increased to 50%, melting point decreased, suggesting formation of form IV. Why was this? Water is a better conductor of heat, and so higher water percentage lead to a faster heat transfer causing too fast of a crystallization.
The 40% fat replacement level was fixed in the subsequent experiments.
Effect Of The Sugar Concentration And The Units’ Temperature
Using an emulsion of 40% water, and working at 30°C in the A unit (Figure 1) the the addition of sugar in the water phase was studied fixing it initially at the concentration of 10% of the water phase (run #5, Table 1).
This resulted in an increase in droplet size compared to run #3, due to increased viscosity of the aqueous phase. And with a melting point of 27.8, it appears the fat crystalized in form IV not the ideal V. The addition of sugar the microstructure, and the sugar itself crystalizes as well. To prevent droplet coalescence, the processing temperature was deceased by 5°C in both units A and C. The result was a decrease in droplet size and melting point going up to 31.3°C. When the units are run below 35°C, it appears the emulsions melting point ranges from 30-34°C, so likely forming the ideal polymorph V.
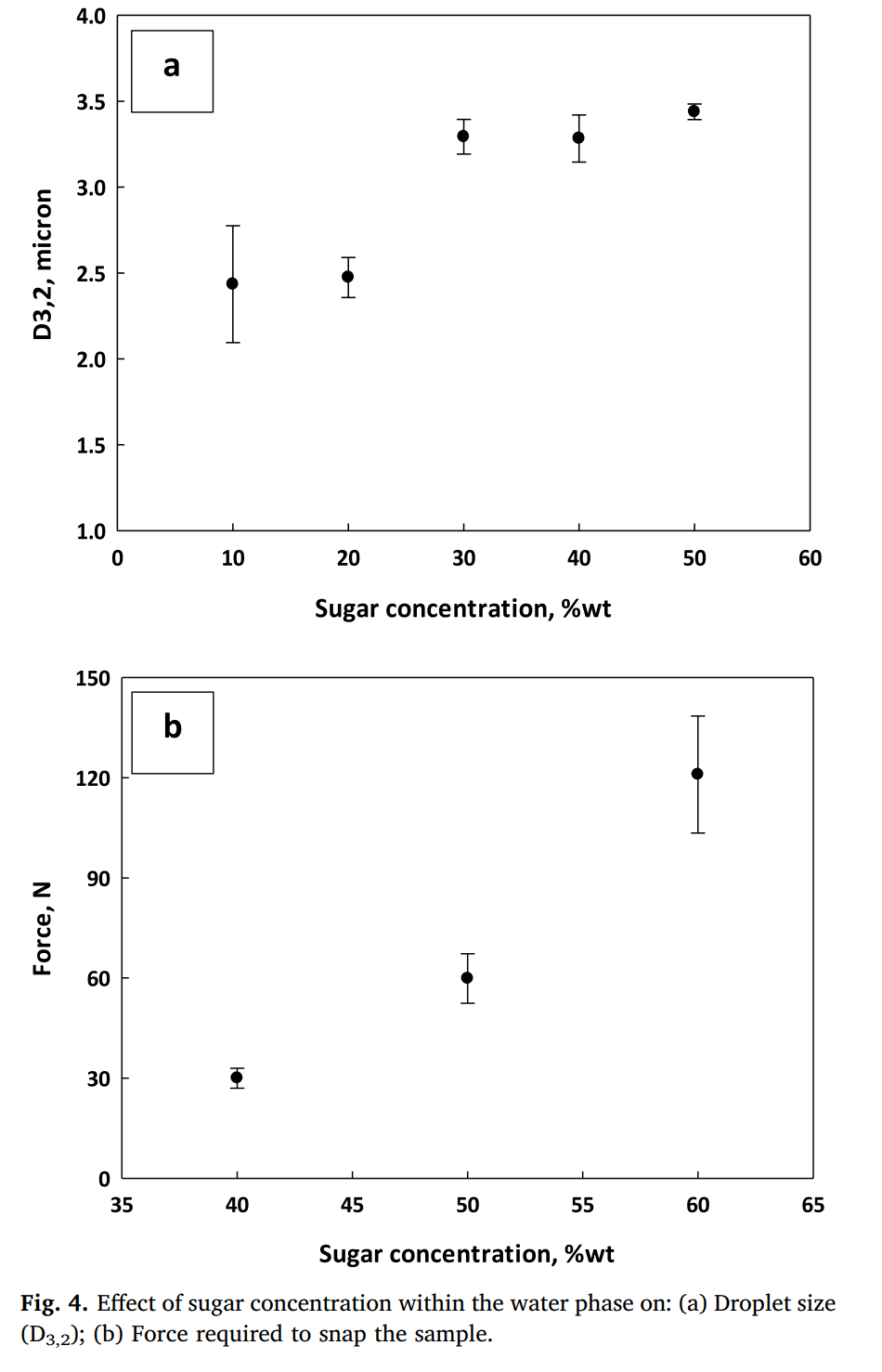
Next, the sugar concentrations were raised up to 60% of the water phase to see the maximum of sugar that could be added to support a strong emulsion (runs 7-11 in Table 1). Increasing sugar lead to larger droplet size (Table 1 and Figure 4a), but still below the threshold of 10 microns (so still too low to be detected by the mouth). Overall, the droplet size and melting behaviour was not affected in any major way with the further additions of more sugar.
Droplet size is important in regards to textural properties of the chocolate. The smaller the droplets, the smaller their effect on product texture. Droplet size was controlled here by adjusting the formulation in terms of water fraction and sugar concentration. Droplet size may be reduced even more by increasing the rotational speed of the units, but this may also break the emulsion.
Full Chocolate Preparation
Full chocolate was made using emulsions containing 40, 50, and 60% sugar in the water (runs #9 10, and 11 respectively, Table 2). The chocolate bars were analyzed in terms of:
Hardness
Thermal behaviour
Water Activity
The first experiments used the emulsion containing 40% sugar (runs #12-16, Table 2) in the water. Stirring was fixed at 300 rpm and the temperature of the jacketed vessel varied according to what was suitable for the mixing of ingredients. At 35°C (run 12 in Table 2), the emulsion broke due to cocoa butter melting allowing the shelled water spheres to release the water. The temperature was then decreased to 30°C (run 13, Table 2), and better mixing was observed, but a large leakage was still visible. The temperature was then reduced again to 25°C (run 14), and this was a good temperature for mixing and maintaining the emulsion. Cocoa butter shells containing water remained intact.
At the same temperature of 25°C, experiments were conducted using the different stirring speeds (runs 15 & 16). In both cases, the result was unsuccessful. When the stirring was too slow, fat crystallization occurred before ingredients could be added, and if stirring was increased the emulsion broke. Sample 14 had low snap and higher water activity compared to the control. It was suggested the mixing worked out well, but microscopically there required some improvements such as a more robust fat shell around the water droplets.
Less sugar needs to be added in the emulsion to make the chocolate, and higher amount of emulsion is used to make mixing easier. At 50% sugar in water (run 17) there was a slight improvement with snap, and there was less water activity (leakage), but still not as good as the control. At 60%, the results showed an even more significant improvement in terms of hardness (Figure 4b), and reduction in water activity. This is also acceptable since at 0.6 sugar content, no microbial proliferation can occur (de Bruijn et al. 2016; Stevenson et al., 2015).
A 40% fat less chocolate with characteristics comparable to commercial chocolate was produced. The use of an industrial mixer or development of an improved mixing system can lead to more successful combination of the ingredients while maintaining a good emulsion.