Effect of sugar, cocoa particles, and lecithin on cocoa butter crystallization
The following is a summary of the stated research mentioned above. The content summarized here, including the figures and tables, all belong to the researchers (unless otherwise indicated). The summary attempts to stay as close to the original paper as much as possible with some adjustments in regards to jargon, length, or to focus on bean to bar aspects.
Introduction:
Fat bloom is an issue in regards to product quality when it comes to chocolate. It can appear as a greyish haze on the chocolate surface. The exact mechanism behind what causes fat bloom is debated. It is believed to be connected with the migration of less stable (liquid) triacylglycerols (TAGS) to the surface of the solid chocolate bar (which may be coincide with the transition between polymorphic forms in cocoa butter.
Fat bloom can be impacted by factors such as:
The temperature chocolate is stored at
The fraction of liquid fat in the chocolate
The addition of milk fat (from the milk powder)
The relationship between tempering (pre-crystallization and cooling) and chocolate microstructure has been evaluated for its impact on shelf life of chocolate. A more dense crystal structure of the cocoa butter within the solid chocolate will result in less fat bloom. This can be obtained through optimal processing (AKA tempering) techniques.
No systematic study exists on the impact of the pre-crystallization process in relation to the various chocolate ingredients on the cocoa butter crystallization and microstructure.
Cocoa Butter Crystals
Cocoa butter can crystalize into 6 different crystalline or polymorphic forms. These forms are denoted by Roman numbers (I-VI) and Greek letters (α, β, β’). Each of the six forms exhibit different melting points and some impact the physical appearance of the chocolate. For commercial chocolate production, only the last three forms (β’IV, β’V, and β’VI) are important, with form β’V being the ideal polymorph. Form β’IV is found in untempered chocolate, and form β’VI is found in bloomed samples.
Achieving form β’V is crucial, as it has the capacity to trap liquid oil within its crystal network, thereby obstructing the migration of liquid TAGs to the surface. If the liquid TAGs reach the surface, then solidify. Since cocoa butter has an off-white appearance when solid, it creates a whitish “bloom” on the surface of the tempered chocolate. The β’V polymorph also has an ideal melting point of 32-34 degrees Celsius which keeps it solid at room temperature, yet melts slightly below body temperature.
Achieving β’V crystals
The most frequently used technique to achieve β’V crystals in the chocolate is to subject the molten chocolate to a well-defined temperature program under the action of shear (think of shear as sliding or mixing the chocolate). This induces the formation of a small proportion (1-3% in volume) of seed crystals. Once the seed crystals are present, the rest of the liquid fat in the chocolate mixture solidifies around the seed crystals, which induces the correct polymorphic form.
This conventional tempering has 4 key steps:
Melt the chocolate to 50°C
Cool the chocolate to the point of crystallization at 32°C
Crystallization at 27°C
Melt out unstable polymorphs by bringing the temperature of the mixture up to 29-31°C
Zeng’s Method
In 2000, Zeng presented a new way of pre-crystallization to produce well-tempered chocolate. They would mix 0.2-2% (weight for weight) of cocoa butter crystals in their most stable form β’VI (the last form) into pre-cooled chocolate (Zeng, 2000). This allows for a large number of nuclei present, which provides a basis for the fat crystals to grow. Even though the seed crystals in the cocoa butter are in the polymorphic form β’VI, the liquid chocolate building on it actually forms into the more preferred β’V form (Zeng et al, 2002).
Other chocolate Components
Although it is the fat of the cocoa bean which crystalizes, other components such as the cocoa solids, the sugar particles, and lecithin also affect the chocolate microstructure.
Savage and Dimick (1995) found that certain phospholipids (such as phosphatidylcholine found in the emulsifier soy lecithin) were present in large quantities in cocoa butter with rapid crystal growth compared to samples with slow crystal growth. This indicates the emulsifier influences the rate of crystallization. Bowser (2006) found similar results. The author also found that cocoa powder gave rise to additional nucleation sites, which resulted in faster crystallization.
Bricknell and Hartel (1998) found that model chocolate which contained amorphous sugar proved to be more resistant to visual fat bloom compared to samples made with crystalline sugar. They suggest the more spherical shape of the amorphous sugar allowed for a more compact microstructure.
Techniques to analyze microstructure
Various techniques have been applied to study cocoa butter crystallization:
PLM (Polarized Light Microscopy) used by Kinta and Hartel (2010)
CLSM (Confocal Laser Scanning Microscopy) offers a way to monitor microstructural development at different depths and length scales under dynamic conditions (Rousseau, 2007).
Bowser (2006) used both PLM and CLSM to investigate the impact of emulsifiers and solid particles on cocoa butter crystallization in tempered and non-tempered chocolate. The impact of milk fats has also been evaluated using CLSM, and was found to generate faster nucleation and crystal growth (Tietz and Hartel, 2000).
Focus of this research
This study aims to clearly understand the microstructure formation under the CLSM in seeded versus non-seeded samples by studying the effect of chocolate ingredients (sugar, cocoa particles, and lecithin) on the kinetics of cocoa butter crystallization. The crystallization was divided into two parts:
Nucleation
Crystal Growth
These both have an impact on the kinetics of cocoa butter crystallization. In order to best investigate this, different chocolate model systems were developed, where adding one ingredient at a time to cocoa butter followed by pre-crystallization. The melting curves of model systems were also measured. Therefore, the relationship between microstructure, crystallization process, and ingredients was investigated.
Materials & Methods:
In order to exclude the effects of particle density (since sugar particles are present in larger quantities than cocoa particles in commercial chocolate), the mass ration between cocoa butter and solid particles were set to a 2:1 ratio in all chocolate model systems. Model systems were composed of:
Pure cocoa butter (100 wt%)
Cocoa butter (66.7 wt%) mixed with sugar particles (33.3 wt%)
Cocoa butter (66.7 wt%) mixed with cocoa particles (33.3 wt% where 10-12% of this is also cocoa butter)
Cocoa butter (66.7 wt%) mixed with a combination of sugar particles (16.65 wt%) and cocoa particles (16.65 wt%)
All samples were prepared in the presence or absence of emulsifier (lecithin 0.5 wt%).
Pre-crystallization and Nucleation
All samples were subject to one of two pre-crystallizations: seeded (by addition of seed) or non-seeded (by temperature treatment).
Seeded
Samples were maintained at 49°C for 20 minutes while being continuously stirred to erase all previous crystal memory. Sample was then brought down to 34°C, and held for 3 minutes before seeds (1 vol%) were added. Samples were kept at 34°C for an additional 2 minutes while being stirred by hand. The temperature was then reduced to 14°C and maintained at this level for up to 60 minutes to induce crystal growth.
Non-seeded
Samples were kept at 47°C for 10 minutes, and then brought down to 27°C and held there for 5 minutes before the temperature was raised to 31°C for 5 minutes.
Results & Discussion:
Chocolate model systems were produced and investigated with CLSM in combination with image analysis to evaluate the effects of pre-crystallization techniques and chocolate components (sugar, cocoa particles, lecithin) on the crystallization process in cocoa butter. Both the pre-crystallization and the components seemed to have a great impact on the kinetics and morphology of the cocoa butter crystallization. You will see visuals of the different microstructures from various samples taken.
DSC measurements were taken to estimate the amount of stable versus non-stable crystals in the chocolate samples. There was a great difference in microstructure between seeded and non-seeded samples.
Tempering pure cocoa butter only
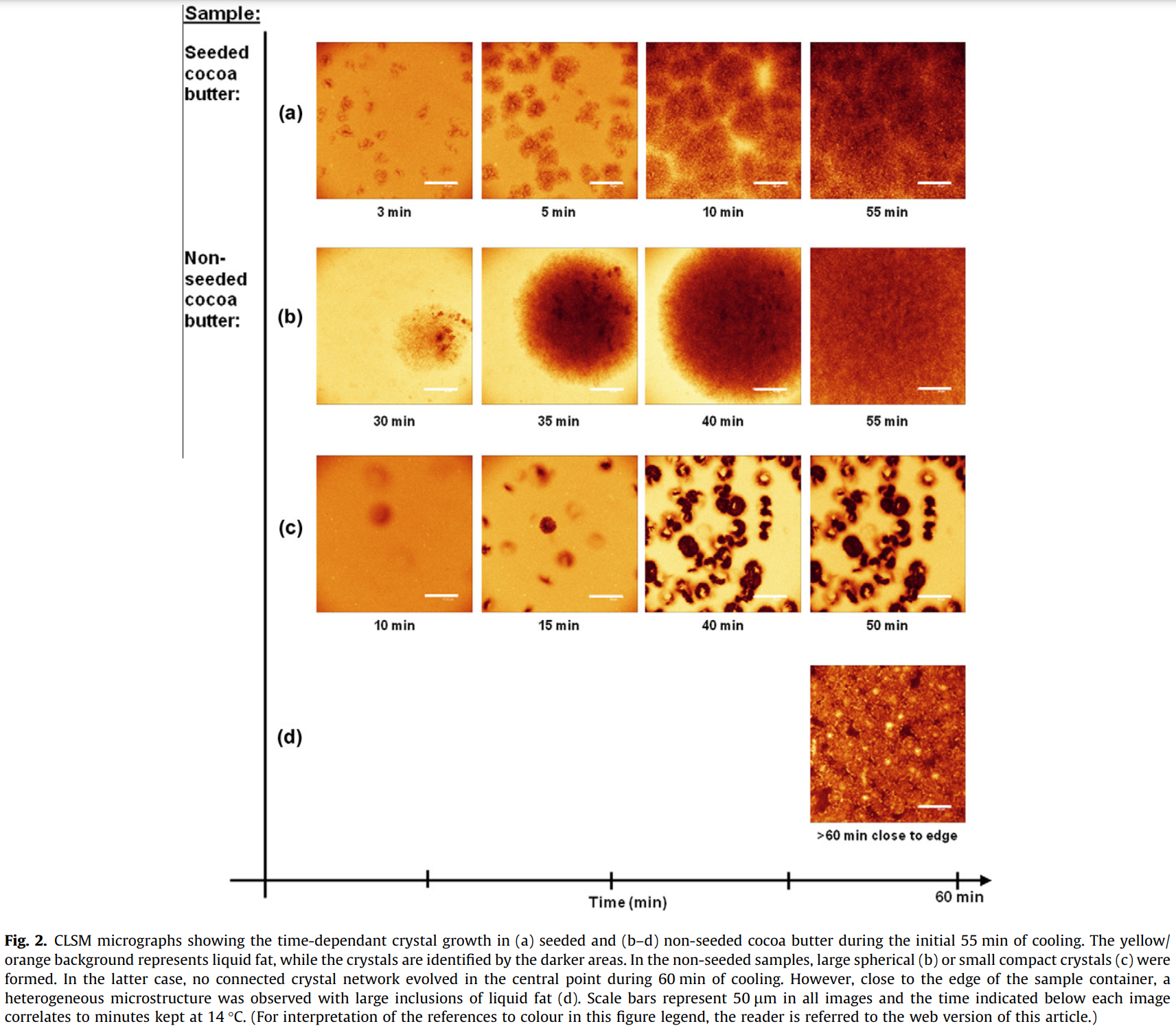
Figure 2 illustrates the time dependent structure evolution of the pure cocoa butter under both seeded and non-seeded pre-treatments. Since the staining dye (BODIPY) had a higher affinity for the liquid cocoa butter compared to the crystals, and therefore were easily contrasted. The lighter yellow/orange represents the liquid cocoa butter, and the darker areas represents the crystals.
The final structures in either the seeded or non-seeded, formed after 60 minutes, were very different. All seeded samples (a) formed multiple nucleation sites, which induced rapid growth of crystals within the first 10 minutes of cooling. The seeds were evenly dispersed throughout the samples, and resulted in a homogenous microstructure with only small proportions of liquid fat trapped in the fat crystal network (which can be seen in the far right image in line (a)).
The non-seeded samples (b-d) shows a more heterogeneous microstructure.
In some cases (Fig. 2(b)) large spherical crystals evolved after an initiation time of 20 minutes, while others (Fig. 2(c)) remained in a semi-liquid state throughout the entire cooling process. As illustrated in Figure 2(c), small compact crystals formed after which no further crystal growth occurred during the 60 minute cooling.
Figure 2(d) illustrates the chocolate microstructure closer to the sample edge as opposed to the central point of the sample (where b and c were taken), and shows us a heterogeneous microstructure with large inclusions of liquid fat connected by large irregular elongated structures penetrating the fat crystals.
Also note that in the non-seeded samples, there was a greater variance in microstructure between samples. CLSM micrographs were analyzed digitally using Analysis Five program (Soft Imaging System GmbH), and so the percentage of crystals in the fat phase in the observed area was able to be estimated.
Figure 3(a) shows that the seeded cocoa butter crystal growth occurred in the first 20 minutes of cooling. The non-seeded crystal growth rates varied and crystal growth was significantly retarded. The large variation in microstructure within the non-seeded samples was supported by DSC analysis. An estimation of the amount of crystals represented by “energy required to melt per gram of fat” is presented in Figure 4(a). Non-seeded cocoa butter had much higher amounts of unstable crystals (at 23-28°C), and smaller quantities of stable crystals (at 31-34°C). The seeded samples had a higher amount of crystals, and the stable crystals were present in larger numbers.
Keep in mind that chocolate has a low thermal conductivity, and so movement of heat through it is slow. Therefore, it’s easier to disperse seeds than to obtain an even temperature distribution in chocolate. The results found in this study support this theory. Non-seeded samples had few nucleation sites and slow crystal growth compared to seeded samples.
Tempering cocoa butter mixed with sugar
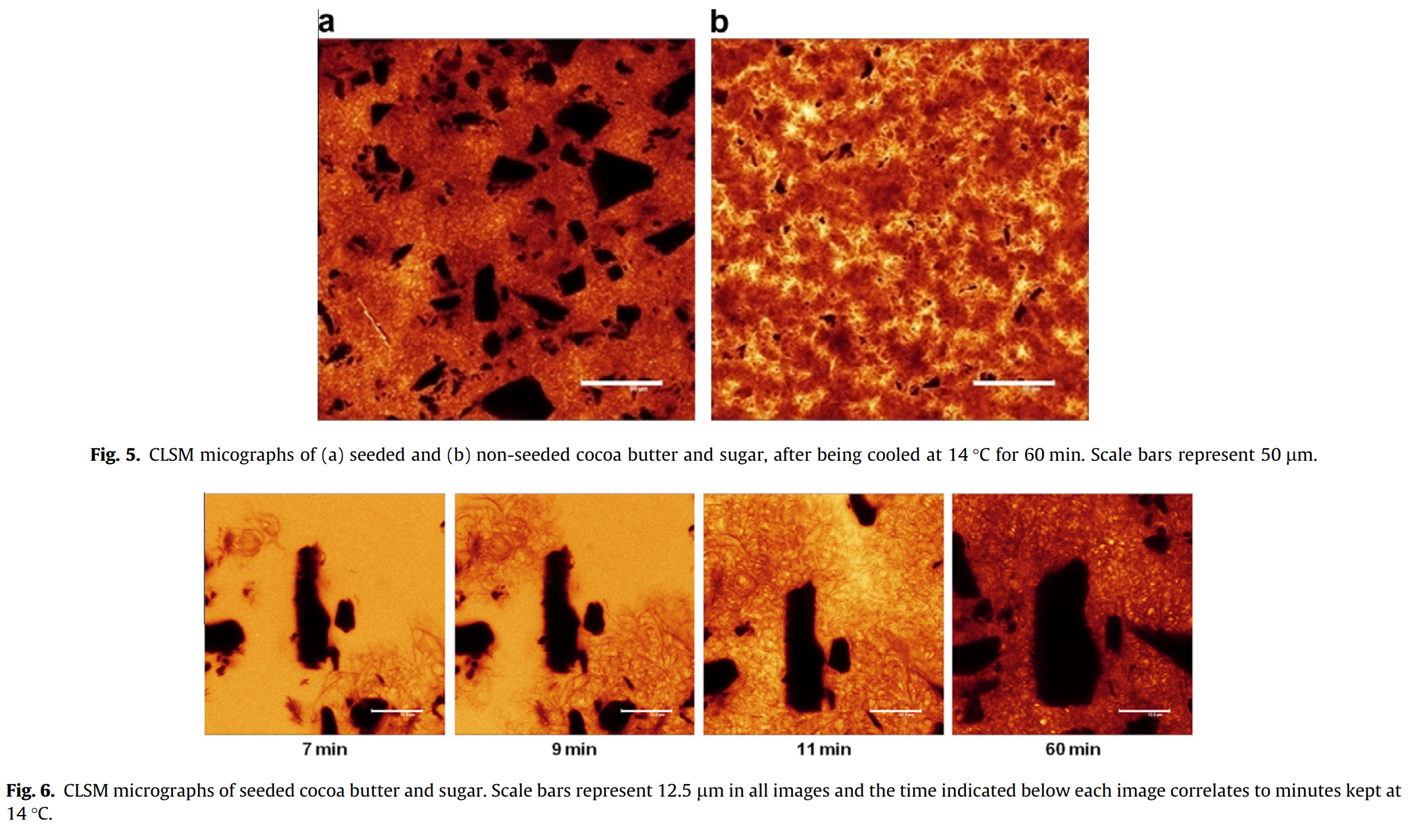
In these samples, cocoa butter was mixed with sugar only. The microstructure of these samples exhibited the same properties as those above made with only pure cocoa butter. The seeded samples had multiple nucleation sites, while the non-seeded samples showed large spherical crystals or small compact ones with severe heterogeneous microstructures as a result (See Figure 5). The sugar appears as black angular shapes, while the fat is displayed as either yellow (liquid) or dark orange (crystal). Figure 5(b) shows the more heterogenous crystal structure with elongated domains with lots of liquid (yellow) cocoa butter trapped between them.
The sugar crystals appeared to have little effect on crystal growth rate in seeded samples compared to the seeded pure cocoa butter samples (Fig. 3(a and b)). However, in the non-seeded sample, sugar appeared to have perhaps enhance crystal growth (Fig. 3(a and c)). This was noted by Dhonsi and Stapley (2006) as well, where a faster shear-induced crystallization was observed when sugar was added to cocoa butter compared to using only the cocoa butter. The authors suggest the idea that perhaps the sugar crystals provide sites for heterogeneous nucleation. Heterogeneous nucleation is a phenomena where foreign surfaces act as nucleation sites for crystallization. However, in this study no micrographs showed fat crystals originating from the plain surface of the sugar in either seeded or non seeded samples (Figure 6).
Tempering cocoa butter mixed with cocoa particles
Here cocoa butter samples were mixed with cocoa particles (which also themselves contain a small amount of fat). In regards to non-seeded samples, cocoa particles did seem to have an impact on the morphology and growth rate of the crystals. Figure 7 displays the micrographs for both seeded and non-seeded. The dark irregularly shaped areas represent the cocoa particles.
The non-seeded samples (Fig. 7(c)) formed small rod-shaped crystals indicated by the circle which contrasts greatly the microstructure in the seeded sample (Fig. 7(a)). The rod-shaped crystals resulted in a heterogeneous microstructure with a large amounts of trapped liquid fat. In order to determine that these rod shapes in Fig. 7(c) were indeed fat crystals and not other by-products from the cocoa solids (cellular membranes, polysaccharides, or proteins), additional non-seeded samples were produced. In these samples, CLSM was used early on in the final part of the con-seeded crystallization (at 31°C), to see if the rods were present before dropping the temperature to 14°C. The samples where rods were discovered were also re-melted to 50°C and no rods were observed either at 31°C prior to crystallization or during the re-melting to 50°C, confirming these rods were fat-based and did not originate from other cocoa particle components. Since cocoa particles contained about 10-12% fat, one could hypothesize that the seeds that were inducing the formation of the rod-shaped crystals were dissolved fatty acids originating from the cocoa particles.
The results shown in Figure 3 indicate that regardless of the pre-crystallization technique, samples containing cocoa butter and cocoa particles had a faster crystal growth rate than the samples with cocoa butter and sugar. This was also found by Bowser (2006), where defatted cocoa particles had an impact on the rate of crystal growth in insufficient pre-crystallized cocoa butter.
As well, DSC measurements showed that there were more stable crystals within the temperature interval of 31-34°C for samples containing cocoa butter and cocoa particles versus cocoa butter and sugar (Figure 8).
Heterogeneous nucleation (the initiation of crystallization on foreign surfaces) is dependent on three interfacial free energies:
Liquid-crystal
Liquid-surface
Crystal-surface
Therefore, one explanation for why the cocoa particles enhanced crystal growth could be that the surface of the cocoa particles is more hydrophobic (water hating) and thereby making it more likely for the heterogeneous nucleation from their surface. However, just as with the sugar crystals, in this study no micrographs showed fat crystals originating from the surface of the cocoa particles in either seeded or non-seeded samples.
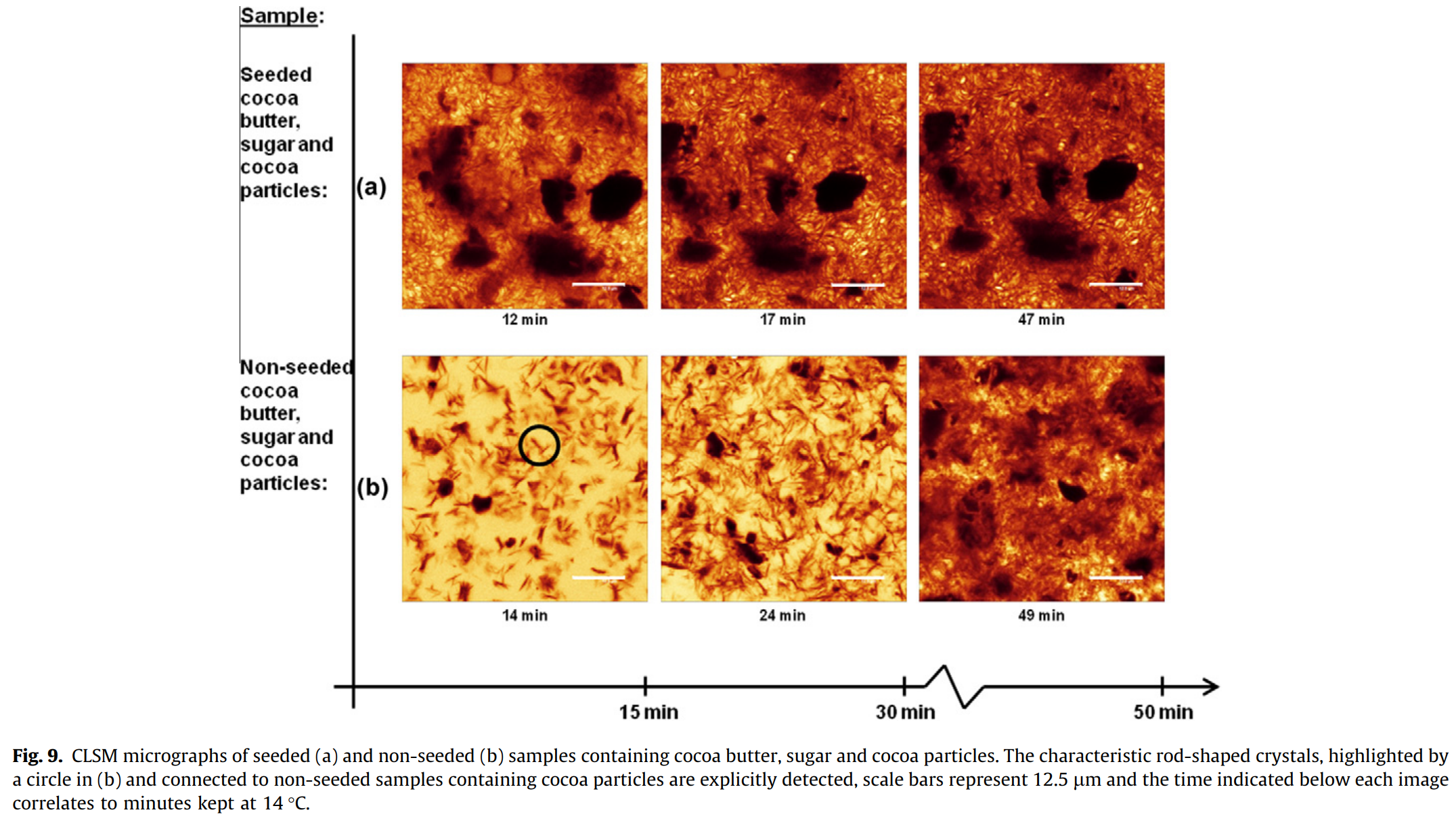
Tempering cocoa butter mixed with sugar and cocoa particles
Figure 9 displays the micrographs of samples containing both sugar and cocoa particles mixed with the cocoa butter. The sugar crystals are completely black, versus the darker less defined areas which represent the cocoa particles (Fig. 9(a)), which were still darker than the fat crystals which are represented by the dark orange.
Although not significant, the crystal growth rate for these samples was slightly slower than the samples that contained only cocoa butter and cocoa particles in both seeded and non-seeded samples. This is not entirely unexpected since the samples containing only sugar and cocoa butter had a slower rate of crystal growth. Therefore, the sugar appears to have a similar influence in these samples as well.
Tempering chocolate with lecithin added
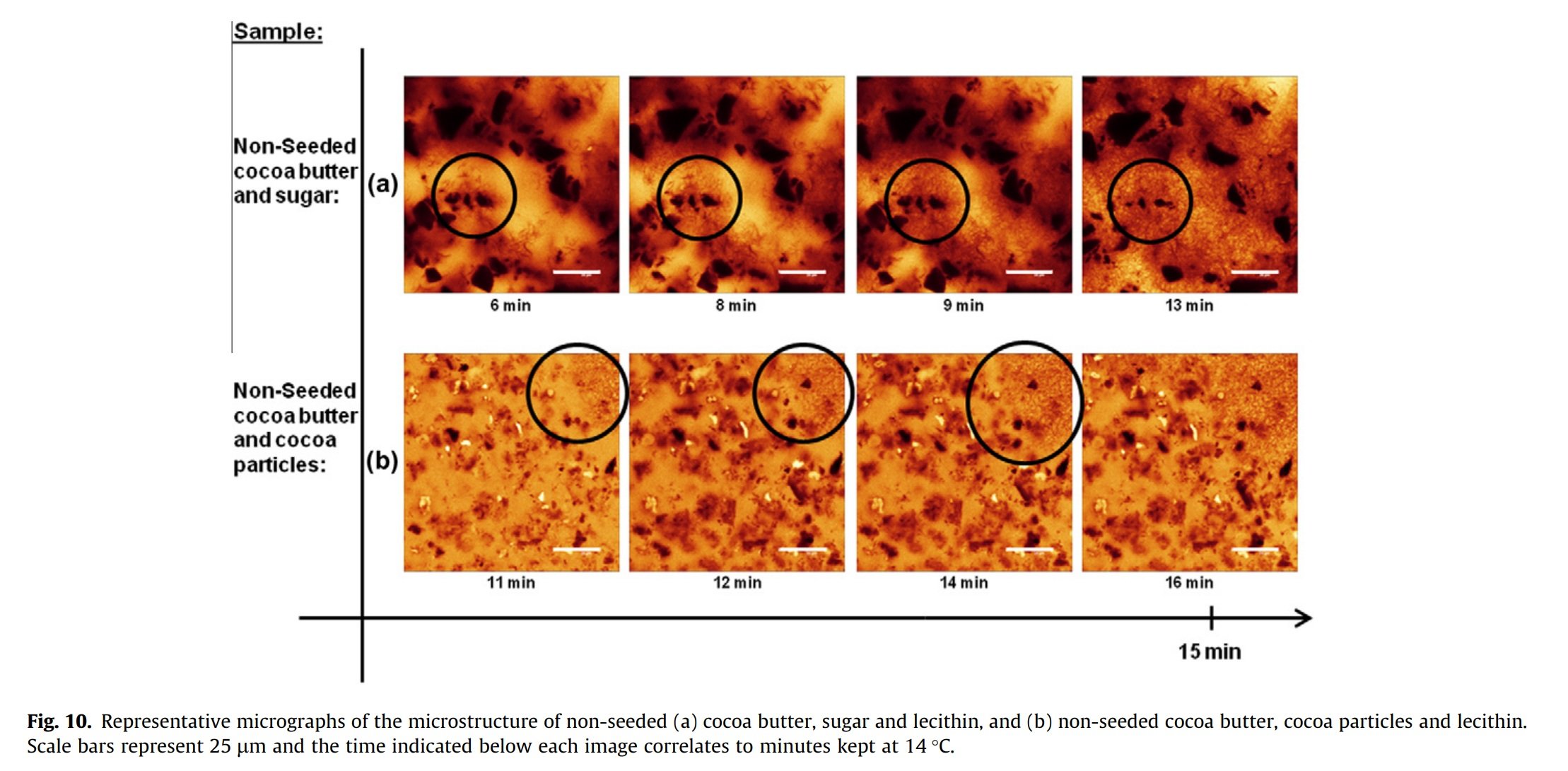
Lecithin appeared to have a pronounced impact on the kinetics of cocoa butter crystallization for both the seeded and non-seeded samples, and for the seeded samples containing cocoa butter and sugar. It was also possible to distinguish a heterogenous nucleation where fat crystals formed from the sugar particle surface (Figure 10(a)). Image analysis also showed a significant increase in crystal growth rate compared to corresponding samples without lecithin (Fig. 11(a)).
Lecithin is found between fat and sugar particles. Lecithin’s lipophilic (fat loving) tail faces the fat phase while it’s lipophobic (fat hating) head faces the sugar. Based on the theory of heterogeneous nucleation, this creates a more favorable environment for the fat crystals to form from, as opposed to forming directly on the hydrophilic/lipophobic surface.
Lecithin also appeared to have an effect on the microstructure in the samples containing only cocoa particles and cocoa butter. Figure 10(b) illustrates non-seeded samples with cocoa particles and lecithin forming rod-shaped crystals at first. However, after about 15 minutes at 14°C a homogenous fat network formed, observed at the top right of the micrographs (see the circle in Fig. 10(b).
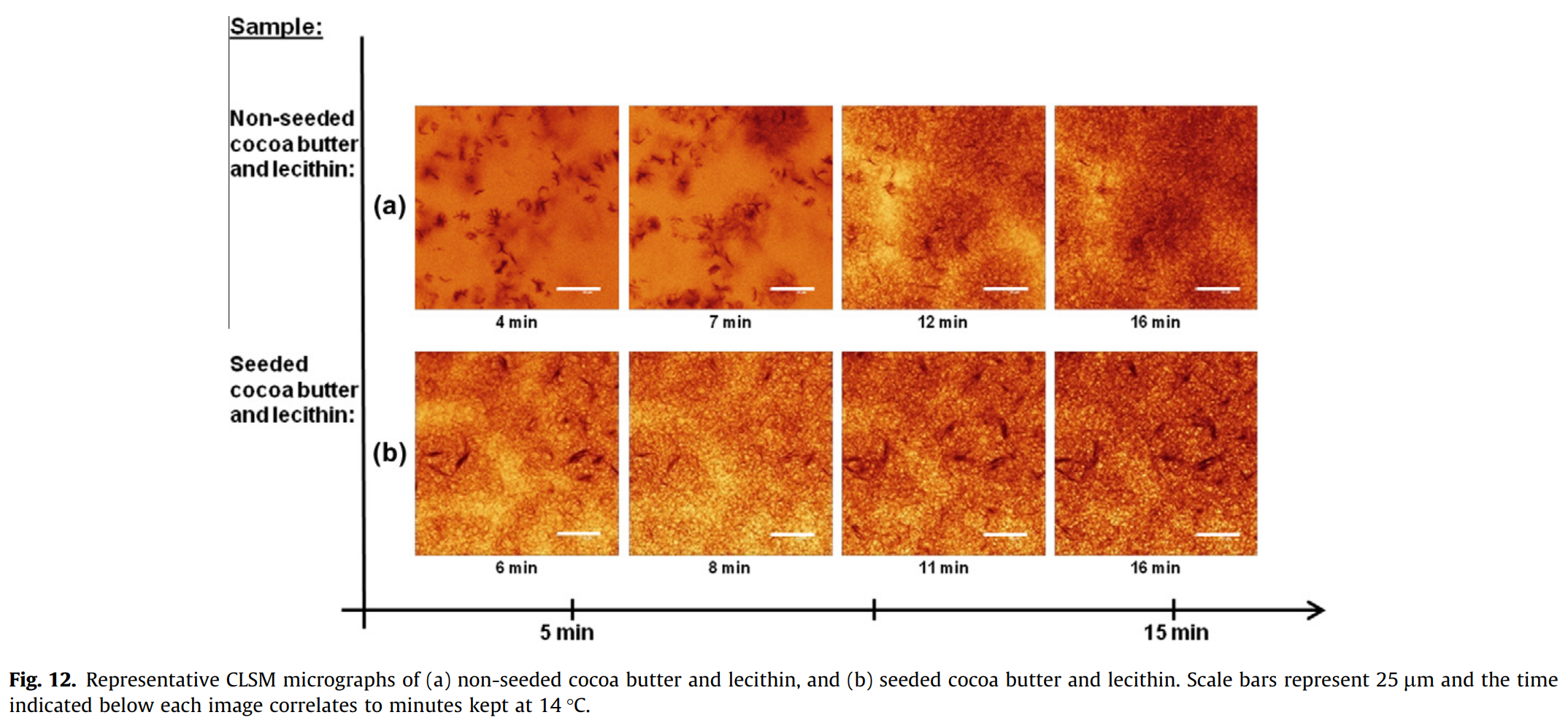
To further understand the effect of lecithin on the kinetics of crystallization, more samples were produced where lecithin was added to pure cocoa butter and subjected to pre-crystallization. In the non-seeded pure cocoa butter, the lecithin itself acted as the seeding material (Fig. 12(a) where you can see small irregular spots from which the fat crystals develop). Compare this with the micrographs in Fig. 2(b-d) of non-seeded pure cocoa butter without lecithin, as well in Fig. 11(c).
Lecithin has an impact on both morphology and crystal growth rate. With the lecithin added, the non-seeded cocoa butter appeared to resemble the seeded cocoa butter with rapid crystal growth and numerous nucleation sites. In seeded pure cocoa butter, it was not possible to distinguish any significant effect from the addition of lecithin, since they already contained seeding materials (Fig. 12(b)).
There have been other reports of the seeding effect of PC (phosphatidylcholine) which is a phospholipid that makes up 15% the total fat content of lecithin. Since it has the tendency to form micelles in the HII mesophase (with polar heads facing the center, and non-polar parts oriented towards the exterior), it allows for a good foundation for the nucleation of fat crystals (Garti and Sato, 2001; Savage and Dimick, 1995). This suggests that the seeds inducing crystal growth in the non-seeded samples (Fig. 12(a)) are micelle structures of PC. However, other reports have contradicted these findings stating that lecithin delays cocoa butter crystallization (Dhonsi and Stapley, 2006).
For what was studied here, it can be concluded that lecithin (at a fraction of 0.5%) had a significant impact on crystal growth rate. The DSC measurements give additional indication of crystal polymorphs generated (see Figure 13(a)). Here one can see lecithin had an impact on the generation of higher stable β-polymorphs. It is unlikely that lecithin preferably nucleates higher stable fat crystal polymorph forms, but a more intensive nucleation and growth of fat crystals during cooling will provide more time for lower-melting polymorphs to transform into a higher stable form (α-form).
Conclusion
CLSM, DSC and image analysis were found to be a good way to evaluate the kinetics and morphology of cocoa butter crystallization in chocolate. Both the pre-crystallization technique (seeded or non-seeded) and the ingredients hand a pronounced impact on the crystallization and microstructure of all chocolate model systems.
Seeded samples formed multiple nucleation sites, which induced a rapid growth of crystals. This resulted in a more homogenous microstructure. The non-seeded samples displayed a more random structure, with some areas developing large spherical crystals while other parts gained a more heterogenous microstructure with large areas of liquid fat and small compact crystals.
Non-seeded samples containing cocoa particles induced the formation of rod-shaped crystals and resulted in a heterogeneous microstructure. For either seeded or non-seeded, sugar appeared to prolong the nucleation and growth of cocoa butter crystals as compared to cocoa particles. However, lecithin greatly enhanced the crystal growth rate in seeded and non-seeded samples with cocoa butter and sugar. Further studies are required to establish whether the effect of lecithin remains in commercial chocolate systems.